To make it possible for cellular agriculture—the process of growing meat in bioreactors—to feed millions of people, muscle cells from chickens, fish, cows, and other food sources will have to be grown to produce millions of metric tons per year. Normal muscle stem cells drawn from live animals to start a culture typically divide only about 50 times before they are no longer viable. That’s not enough to provide the amounts that will be needed to fuel the cellular agriculture industry sustainably.
Searching for a solution, researchers at Tufts University Center for Cellular Agriculture (TUCCA) developed immortalized bovine muscle stem cells (iBSCs), which can grow rapidly and divide hundreds of times—and possibly indefinitely. This advance means that researchers and companies around the globe can have access to and develop new products without having to source cells repeatedly from farm animal biopsies.
Two steps were key to transforming regular bovine muscle stem cells into the immortalized bovine muscle stem cells:
Most cells, as they divide and age, begin to lose DNA at the ends of their chromosomes (called telomeres), like worn ropes that get frayed with use. This can lead to errors when the DNA is being copied or repaired. It can also cause genes to be lost and, eventually, cells to die. The Tufts researchers engineered the bovine stem cells to constantly rebuild their telomeres, effectively keeping their chromosomes youthful and ready for another round of replication and cell division.
The second step in immortalizing the cells was to make them continuously produce a protein that stimulates a critical stage of cell division. This effectively turbocharges the process and helps the cells to grow faster.
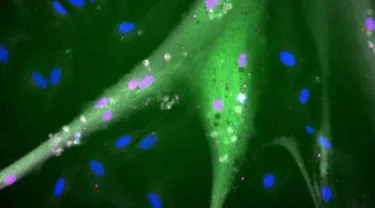
Muscle stem cells are not the final product that one wants to eat. They must not only divide and grow but also differentiate into mature muscle cells just like (or at least very similar to) the muscle cells in a steak or filet. The research team found that the new stem cells did indeed differentiate into mature muscle cells—although these are not entirely identical to animal muscle cells or muscle cells from conventional bovine stem cells. They are exploring further how to engineer the cells to differentiate more optimally.
Immortalized cells developed by the TUCCA team offer several advantages over conventional stem cells. One is the possibility of producing significantly more mass for meat production. Another is lowering the barrier of entry for other researchers to explore cellular agriculture, thus expanding the number of researchers engaged in finding ways to reduce costs and overcome challenges to scaled-up production.