Known for its role producing serotonin in the brain, new evidence shows that expression of the enzyme tryptophan hydroxylase 2 (TPH2) in fat cells also has an important role in the metabolic complications associated with obesity. A new study from researchers at the Jean Mayer USDA Human Nutrition Research Center on Aging (HNRCA) and Tufts University School of Medicine and colleagues found that expression of TPH2 is upregulated in the adipocytes (fat cells) of people with obesity, contributing to systemic downstream effects.
Andrew Greenberg, senior author on the paper and senior scientist at the Metabolism and Basic Biology of Aging directive at HNRCA, has spent his career investigating obesity and the health problems that result from it, a lifelong interest motivated by his family’s history of obesity-related health diseases.
“The goals of our laboratory are to determine the mechanisms that cause obesity and its metabolic complications; to dissect the pathways that regulate obesity and its metabolic complications; and, hopefully, to use those pathways to develop novel therapies,” said Greenberg.
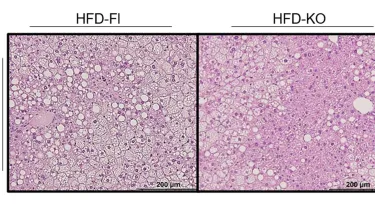
The study, published in the Journal of Clinical Investigation, teased out TPH2’s role in obesity using genetically engineered mice. Greenberg and his team developed mice whose fat cells do not produce TPH2 and fed them a high-calorie diet to induce obesity. Although the mice still gained weight, they were better able to metabolize insulin and glucose, and they accumulated less fat in the liver than mice with normal TPH2 expression in their fat cells.
When blood sugar is high, insulin normally directs the liver to stop producing glucose and start storing it. In obesity, the liver becomes resistant to insulin, leading to persistently elevated blood sugar and a predisposition to diabetes.
The Greenberg laboratory observed additional effects in the TPH2-deficient mice in the form of slowed digestion. Serotonin quickens the movement of food through the digestive system, but without TPH2, the resulting shortage of serotonin led food to move slower, giving the intestine more time to absorb calories. These extra calories balanced out the effects of their increased energy expenditure, meaning they didn’t end up losing fat.
“Even though body fat was not reduced, TPH2 deficiency was still beneficial to metabolism, which is consistent with the observation that body fat by itself is not necessarily bad,” Greenberg said.
Next, the team investigated the opposite scenario: an abundance of TPH2 in fat cells. They developed mice that overexpressed TPH2 just in one fat storage area, or depot, in the abdomen. The accumulated TPH2, however, had effects on distal tissues far beyond that one fat depot.
“[In mice with TPH2 overexpression], serotonin, to our surprise, was secreted in the blood and altered fat cell metabolism, reduced energy expenditure, and increased body fat and liver fat accumulation. Serotonin, secreted by TPH2, thus had the characteristics of a hormone because of its effects on distal organs,” Greenberg explained.
To test whether these findings translate in humans, the team collaborated with Michael Jensen and colleagues at the Mayo Clinic to perform similar experiments in human fat cells. In obese human fat cells, TPH2 levels were indeed increased—and found to have detrimental systemic effects on metabolism. The increased TPH2 levels were also associated with liver fat accumulation, insulin resistance, and increased levels of serotonin in the blood.
“Obesity is the major cause of fatty liver, and fatty liver is the major cause of liver dysfunction and liver cancer in the world now,” Greenberg noted.
By seeing what happens when expression of TPH2 is increased in fat cells, the researchers found that TPH2 plays a decisive role in the cascade of complications that result from obesity.
“In obesity, the TPH2 made in fat cells secretes serotonin, which detrimentally alters fat cell metabolism and is secreted into the blood, where it goes to the liver and other tissues to worsen insulin resistance and increase liver fat,” Greenberg said.
Through this cycle, the elevated insulin levels that accompany obesity generate even greater insulin resistance and a predisposition to diabetes, exacerbating obesity’s metabolic complications.
With this novel understanding of the metabolic mechanisms at play in obesity, the researchers hope to uncover ways of reducing TPH2 levels in people with obesity, lessening downstream health problems.
Citation: Park BI, Reeves AR, Zhu Y, Wilson RA, Fernandes SC, Buhman KK, Lytle KA, Jensen MD, Greenberg AS. J Clin Invest. 2025;135(14):e190765. DOI: https://doi.org/10.1172/JCI190765.